H 89 2HCl
CAS No. 130964-39-5
H 89 2HCl( 5-Isoquinolinesulfonamide | Protein Kinase Inhibitor H-89 )
Catalog No. M11238 CAS No. 130964-39-5
H 89 2HCl is a potent PKA inhibitor with Ki of 48 nM, 10-fold selective for PKA than PKG.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 30 | In Stock |


|
| 10MG | 48 | In Stock |


|
| 25MG | 97 | In Stock |


|
| 50MG | 192 | In Stock |


|
| 100MG | 327 | In Stock |


|
| 200MG | 484 | In Stock |


|
| 500MG | 771 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameH 89 2HCl
-
NoteResearch use only, not for human use.
-
Brief DescriptionH 89 2HCl is a potent PKA inhibitor with Ki of 48 nM, 10-fold selective for PKA than PKG.
-
DescriptionH 89 2HCl is a potent PKA inhibitor with Ki of 48 nM, 10-fold selective for PKA than PKG, greater than 500-fold selectivity than PKC, MLCK, calmodulin kinase II and casein kinase I/II.(In Vitro):H-89 inhibits protein kinase A, in competitive fashion against ATP. H-89 causes a dose-dependent inhibition of the forskolin-induced protein phosphorylation, with no decrease in intracellular cyclic AMP levels in PC12D cells. H-89 significantly inhibits the forskolin-induced neurite outgrowth from PC12D cells. H-89 (30 μM) inhibits significantly cAMP-dependent histone IIb phosphorylation activity in PC12D cell lysates. H-89 (1-2 μM) significantly slows the repriming rate in rat skinned fibres, most likely due to it deleteriously affecting the T-system potential. H-89 (10-100 μM) inhibits net Ca2+ uptake by the SR and affectes the Ca2+-sensitivity of the contractile apparatus in rat skinned fibres.(In Vivo):H-89 (0.2 mg/100g, i.p.) significantly increases seizure latency and threshold in PTZ-treated animals. H-89 (0.05, 0.2 mg/100 g, i.p.) prevents the epileptogenic activity of bucladesine (300 nM) with significant increase of seizure latency and seizure threshold.
-
In VitroH-89 inhibits protein kinase A, in competitive fashion against ATP. H-89 causes a dose-dependent inhibition of the forskolin-induced protein phosphorylation, with no decrease in intracellular cyclic AMP levels in PC12D cells. H-89 significantly inhibits the forskolin-induced neurite outgrowth from PC12D cells. H-89 (30 μM) inhibits significantly cAMP-dependent histone IIb phosphorylation activity in PC12D cell lysates. H-89 (1-2 μM) significantly slows the repriming rate in rat skinned fibres, most likely due to it deleteriously affecting the T-system potential. H-89 (10-100 μM) inhibits net Ca2+ uptake by the SR and affectes the Ca2+-sensitivity of the contractile apparatus in rat skinned fibres.
-
In VivoH-89 (0.2 mg/100g, i.p.) significantly increases seizure latency and threshold in PTZ-treated animals. H-89 (0.05, 0.2 mg/100 g, i.p.) prevents the epileptogenic activity of bucladesine (300 nM) with significant increase of seizure latency and seizure threshold.
-
Synonyms5-Isoquinolinesulfonamide | Protein Kinase Inhibitor H-89
-
PathwayApoptosis
-
TargetPKA
-
RecptorPKA| S6K1
-
Research AreaOther Indications
-
Indication——
Chemical Information
-
CAS Number130964-39-5
-
Formula Weight519.28
-
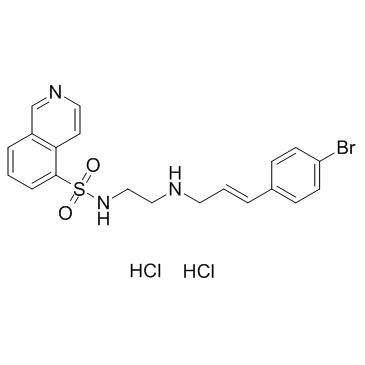
Molecular FormulaC20H20BrN3O2S.2HCl
-
Purity>98% (HPLC)
-
SolubilityDMSO:104 mg/mL (200.27 mM); Ethanol:<1 mg/mL (<1 mM); Water:6 mg/mL (11.55 mM)
-
SMILESC1=CC2=C(C=CN=C2)C(=C1)S(=O)(=O)NCCNC/C=C/C3=CC=C(C=C3)Br.Cl.Cl
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Chijiwa T, et al. J Biol Chem., 1990, 265(9), 5267-5272.
molnova catalog



related products
-
Rp-cAMPS sodium
Rp-cAMPS sodium is a phosphorothioate analog of cAMP, a protein kinase A inhibitor and a membrane-permeable cAMP antagonist that inhibits cAMP-dependent protein kinases by blocking cAMP-induced conformational transitions, and can be used in the study of cardiovascular diseases.
-
Pomolic acid
Pomolic acid has anti-cancer, anti-inflammatory and apoptotic activities.
-
KT-5720
KT-5720 is a highly specific PKA (cAMP-dependent protein kinase) inhibitor with Ki of 60 nM displays little to no activity for MLCK, cGPK, and PKC (Ki>2 uM).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


